Empowerment through Knowledge
NRL is dedicated to improving global infectious disease testing through knowledge sharing and education. Our consulting, training, and mentoring programs emphasise self-sufficiency, sustainability, and cultural sensitivity. We tailor our services to meet the unique needs of each partner, enhancing laboratory and community-based testing. Through initial capacity assessments, we create customised training and mentoring plans that foster long-term, trust-based professional relationships.
View Our Prospectus For More
Overview - What do we do?
-
The scope of NRL’s consulting, training and mentoring activities ranges from:
- Providing one-off technical training or advice to troubleshoot and address specific knowledge gaps, or design and/or verify a new process or program.
- Performing capacity assessments and gap analyses to identify and inform capacity building strategies and workplans.
- Design and delivery of customised training programs including mentoring sessions aimed at addressing specific needs, priorities or challenges, at an organisational or national level. For example, supporting the development of National External Quality Assessment Schemes or supporting an organisation to undertake the journey towards full quality accreditation.
- Delivery of train-the-trainer programs to empower selected local trainers to educate and mentor others.
- Development of guidance documents in collaboration with partners such as the World Health Organization.
NRL provides consultancy, training and, mentoring services in the following areas:- Quality Management Systems, including capacity development towards attaining compliance and/or accreditation to national and international standards (e.g. ISO 15189, ISO 17043 and Codes of GMP).
- External Quality Assessment Schemes (EQAS), including the design and implementation of a sustainable national EQAS to quality assure testing of local laboratories; and reduce the reliance on international, expensive and inconsistent support.
- Test Kit evaluation and supporting reference laboratories to design protocols, maintain appropriate samples and assess key performance characteristics of a test kit at a given point in time; including the use of data to recommend test kits for use in-country, often as part of a regulatory framework.
- Testing Algorithms including support to reference laboratories for the development of a national testing strategy that is appropriate for in-country context, and the design and validation of a testing algorithm through the testing of carefully characterised samples in different assay combinations.
- Laboratory Quality Control
- Point-of-Care Quality Assurance
- Equipment Procurement, Validation and Maintenance
- Biosafety and Biosecurity
- Capacity Assessments
Where do we work?We have significant experience working with Ministries of Health, international development partners, and laboratory and community-based testing personnel in a range of countries.
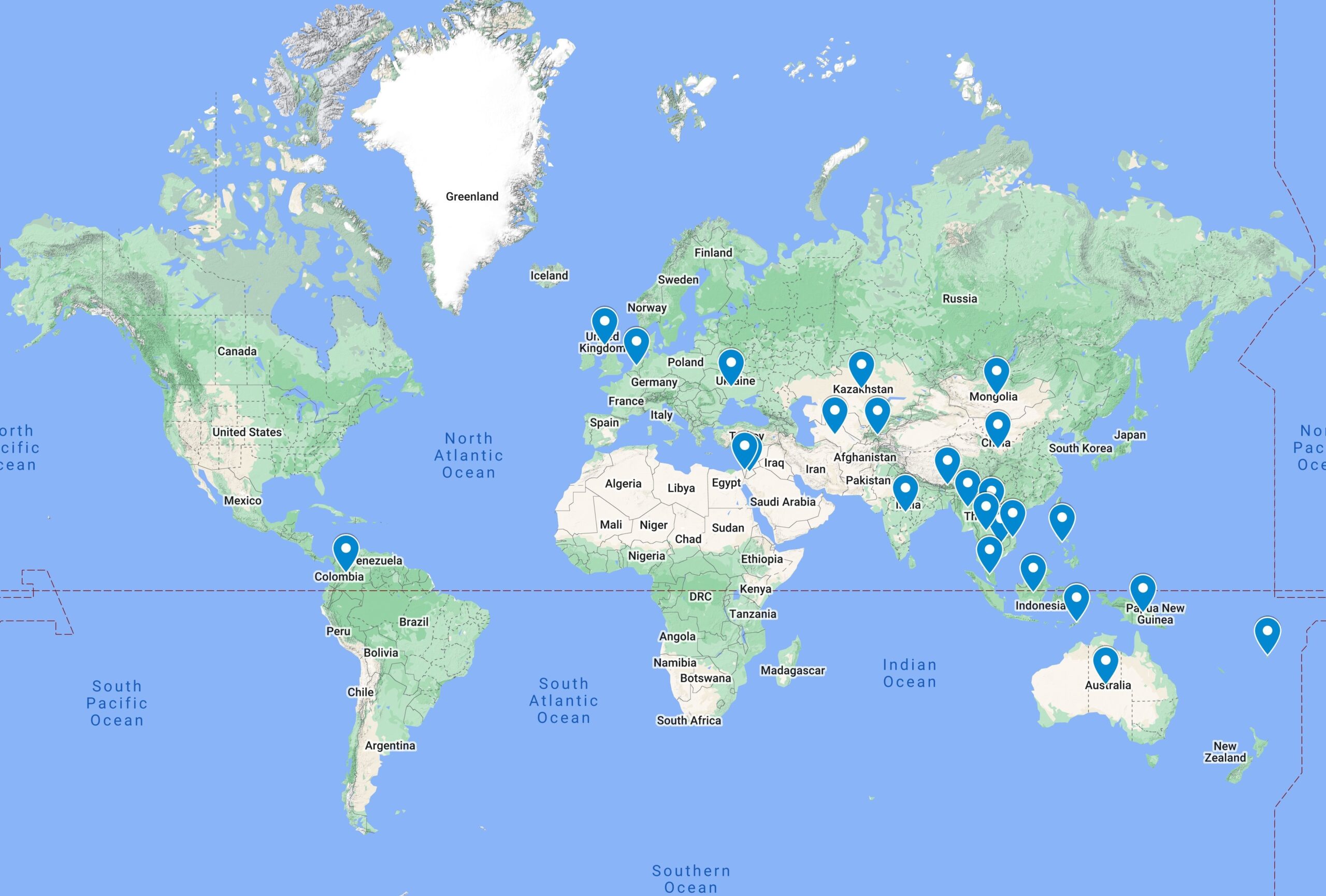
(Cambodia, Laos, Indonesia, Philippines, Myanmar, Vietnam, Mongolia, India, Bhutan, Tajikistan, Turkmenistan,
Kazakhstan, Jordan, Timor-Leste, Thailand, Fiji, China, PNG,Columbia, Israel, Malaysia, Netherlands, Ukraine, United Kingdom, Australia)Current ProjectsNRL is collaborating with the Australia Indonesia Health Security Partnership (AIHSP) and the Indonesia One Health University Network (INDOHUN) to support selected primary (or Puskesmas), district and provincial public health laboratories, in 10 districts spread out over 5 provinces (Central Java, Yogyakarta, Bali, South Sulawesi, East Nusa Tenggara or NTT). The collaboration will improve the quality of their testing systems through the involvement of dedicated trainees who will act as Quality Champions during a series of training and mentoring programs customised to address identified challenges faced by the laboratories. Funded by the Australian Department of Foreign Affairs and Trade (DFAT), the aim of this collaboration is to ultimately strengthen laboratory capacity in both public health and hospital systems in the selected provinces.
AIHSP’s webpage: https://www.aihsp.or.id/
INDOHUN’s webpage: https://indohun.org/
2024 Projects
-
Indonesian Workshop, Session 1 - January 2024
Overview

In collaboration with Australia Indonesia Health Security Partnership (AIHSP) and The Indonesia Health University Network (INDOHUN), the NRL Scientific Consulting and Training (SCT) team have been implementing a laboratory capacity building and strengthening program in Indonesia.
The program focuses on strengthening the implementation of quality management systems (QMS) in primary health, district health and provincial health laboratories in 5 provinces. The goal is to improve the quality of testing in these laboratories and equip them to provide reliable diagnostic testing for both clinical diagnosis and public health surveillance purposes.
Our Scientific Consulting and Training team held their first training workshop in Indonesia – Here’s the updates
In late January, the SCT held the first face-to-face training workshop in Yogyakarta over 5 days. The objectives were to provide an overview of laboratory QMS, focusing on its use in identifying and managing risks to the quality and accuracy of test results and outlining the principles behind the key QMS activities that form the foundation for the QMS. The team also visited some of the laboratories to gain firsthand appreciation of the scope of work, workflows, available resources, and challenges faced by the laboratories.
The workshop was attended by quality champions selected from participating laboratories; observers from the reference laboratories, Ministry of Health, and the Health Training Centre (CILOTO); and representatives from both INDOHUN and AIHSP.
The team appreciates the logistical and technical support provided by both AIHSP and INDOHUN to make the workshop a success.
Indonesian Workshop, Session 2 - June 2024Our Scientific Consulting and Training team held their second training workshop in Indonesia – Here’s the updates

The NRL Scientific Consulting and Training (SCT) team is implementing a laboratory capacity building and strengthening program in Indonesia in collaboration with Australia Indonesia Health Security Partnership (AIHSP) and The Indonesia Health University Network (INDOHUN). The program focuses on strengthening the implementation of quality management systems (QMS) in primary health, district health and provincial health laboratories in 5 provinces. The goal is to improve the quality of testing in these laboratories and equip them to provide reliable diagnostic testing for both clinical diagnosis and public health surveillance purposes.
In late June, the SCT held the second face-to-face training workshop in Tangerang over 3 days. The workshop was attended by quality champions selected from participating laboratories; observers from the reference laboratories, Ministry of Health (MOH), and the health training centre (Balai Besar Pelatihan Kesehatan, BBPK); and representatives from both INDOHUN and AIHSP. The training program for workshop 2 was designed to provide advanced training on topics that had been requested by participants or identified as part of previous activities. As part of the expected training outcomes participants were expected to:
- Improve their understanding of risk assessment processes and creation of risk registers;
- Understand how to identify opportunities for improvement and the importance of doing so;
- Be able to use Training Checklists as part of the design and delivery of training to staff;
- Understand the similarities and differences between good clinical laboratory practice and a QMS based on ISO 15189;
- Improve their knowledge of the management of nonconformities, including the importance of performing root cause analysis;
- Understand the importance of internal audits and how to plan for an internal audit;
- Gain a better understanding of the principles of laboratory biosafety and biosecurity, including the need to perform safety assessments.
The team also held meetings with key stakeholders, including representatives from MOH, BBPK, AIHSP and INDOHUN. The team also appreciates the logistical and technical support provided by both AIHSP and INDOHUN to make the workshop and stakeholder meetings a success.
2023 Projects

2023 Projects
What has the team been up to?Articles

Strengthening Laboratory Testing – A Call for Quality, Innovation, and Systemic Change
Download NowMeet Our Trainers
-
Geraldine Kong, BSc (Econ), MSocSci - Manager - Consulting & Training

Geraldine Kong, Scientific Consulting and Training Manager at NRL, brings over 20 years of experience collaborating with international partners across government and the non-governmental sector. She has worked in partnership with organizations such as the Global Fund, the World Health Organization, the Mérieux Foundation, and the Australia-Indonesia Health Security Partnership. Geraldine specializes in managing and coordinating capacity-building projects focused on Strengthening laboratory quality in Asia, the Pacific, and Africa, overseeing activity plans, budgets, and reporting requirements.
Vee Armstrong, HNC (UK) Medical Laboratory Sciences - Senior Scientist
Vee Armstrong is a Senior Scientist and Consultant with more than thirty years of experience in quality management systems, GMP, and regulatory affairs. She has worked with WHO on the development of Guidance documents for blood services and with APEC on QMS capacity building to identify and establish Centres of Excellence among blood services in Vietnam. Vee has also supported capacity building of clinical and blood testing laboratories in the Asia-Pacific region through virtual and on-site training and assessments.
Innocent Mupunga, PhD (Medical Sciences), MSc, HBMLS - Senior Scientist
Innocent Mupunga is a Senior Scientist and Consultant with over two decades of expertise in laboratory diagnostics, quality management systems, biosafety, biosecurity, and capacity building. I specialize in training laboratory professionals and providing scientific consultancy and advisory services globally. My experience includes leading clinical laboratories, implementing quality and safety systems, and strengthening public health laboratory capacity in Africa and Asia-Pacific regions.







